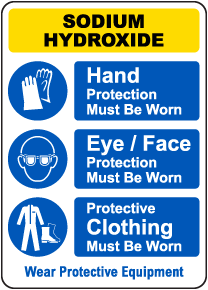
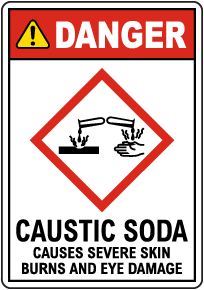
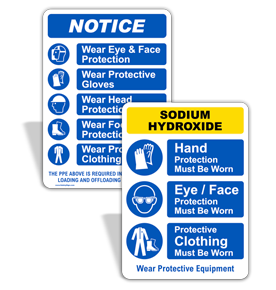
Shop Our Wide Sodium Hydroxide Sign Selection
Custom Sodium Hydroxide Signs
Design your own Custom Sodium Hydroxide Signs. Choose a template that fits your needs, then add your own text, image / logo or upload your artwork. Click on See All for more custom templates.
Other Categories You Might Be Interested In
What is Sodium Hydroxide / Caustic Soda?
Sodium hydroxide, also known as lye or caustic soda, is an alkali inorganic compound - a type of base that can neutralize acids and is soluble in water. Pure sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. As a liquid, is colorless and denser than water. When dissolved in water or neutralized with acid it liberates substantial heat, which may be sufficient to ignite combustible materials.
Sodium hydroxide is commercially available in pellets, flakes, granules and as a 50% saturated solution. Sodium hydroxide is a highly versatile substance used to manufacture soaps, rayon, paper, explosives, dyestuffs, and petroleum products. It is also used in processing cotton fabric, laundering and bleaching, metal cleaning and processing, oxide coating, electroplating, and electrolytic extracting. It is commonly present in commercial drain and oven cleaners.
Due to its strong corrosive qualities, exposures to sodium hydroxide in its solid or solution form can cause skin and eye irritation. Sodium hydroxide can burn or eat away at any part of the body that it contacts. Breathing sodium hydroxide dust or mist causes mild or serious effects, depending on the amount of exposure. Effects may include sneezing, sore throat or runny nose. Severe inflammation of the lungs can occur.
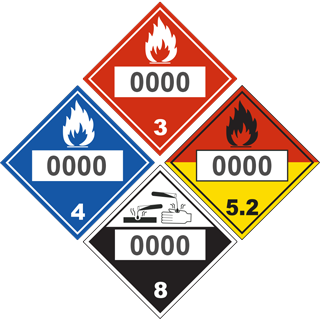
Fire Protection Guide to Hazardous Materials - Edition 14th
| Chemical Name / CAS No. | Health | Flammability | Instability | Special Hazard | DOT Class | ID No. |
|---|---|---|---|---|---|---|
| Sodium Hydroxide 1310-73-2 |
3 | 0 | 1 | None | Class 8 Corrosive Material |
UN 1823 - Solid UN 1824 - Solution |
The signs above represent our interpretation of material information in combination with NFPA 30 and NFPA’s Fire Protection Guide to Hazardous Materials. Without knowledge of your specific chemical, facility, or hazard, it’s impossible for us to guarantee these signs will match your situation. It is your responsibility to decide which signs are valid for your use and to comply with applicable laws and standards. This site is not intended as a substitute for expert analysis or professional consultation. We make no guarantee of the accuracy of the information on this site and assume no liability of injury or damage as a result of using our products.